Which of the following molecules are constitutional isomers – Embarking on a journey into the realm of constitutional isomers, we uncover the intricate world of molecular structures and their profound impact on various scientific disciplines. Constitutional isomers, molecules with identical molecular formulas but distinct structural arrangements, present a captivating challenge in chemistry, demanding a keen eye for detail and a comprehensive understanding of molecular architecture.
As we delve deeper into this captivating subject, we will explore the fundamental concepts of constitutional isomerism, unraveling the secrets behind their unique properties and behavior. Through a meticulous examination of real-world examples, we will gain invaluable insights into the significance of constitutional isomers in fields ranging from drug design to materials science.
Constitutional Isomers
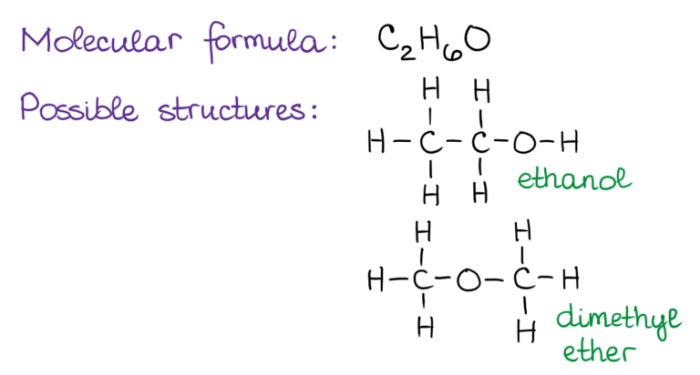
Constitutional isomers are molecules that have the same molecular formula but differ in the arrangement of their atoms. This difference in arrangement can lead to significant differences in the physical and chemical properties of the isomers.
For example, butane and isobutane are both hydrocarbons with the molecular formula C 4H 10. However, butane has a straight-chain structure, while isobutane has a branched structure. This difference in structure leads to differences in their boiling points, with butane boiling at -0.5 °C and isobutane boiling at -11.7 °C.
Constitutional isomers are important because they can have different biological activities. For example, the drug ibuprofen has two constitutional isomers, one of which is active and the other is not.
Identifying Constitutional Isomers
There are a number of ways to identify constitutional isomers. One way is to use molecular formulas. If two molecules have the same molecular formula, they could be constitutional isomers. However, it is important to note that molecules with the same molecular formula can also be structural isomers, which have the same molecular formula but different connectivity of their atoms.
Another way to identify constitutional isomers is to use structural formulas. Structural formulas show the arrangement of atoms in a molecule. If two molecules have different structural formulas, they are constitutional isomers.
Examples of Constitutional Isomers
There are many examples of constitutional isomers. Some common examples include:
- Butane and isobutane (C 4H 10)
- Pentane and 2-methylbutane (C 5H 12)
- Hexane and 2-methylpentane (C 6H 14)
- Heptane and 2-methylhexane (C 7H 16)
- Octane and 2-methylheptane (C 8H 18)
Methods for Distinguishing Constitutional Isomers
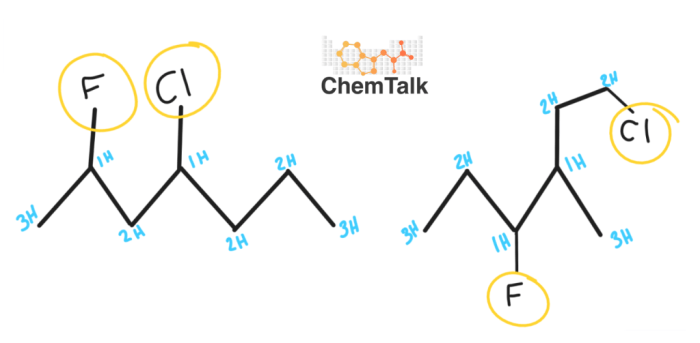
There are a number of methods that can be used to distinguish between constitutional isomers. These methods include:
- Melting point determination
- Boiling point determination
- Infrared spectroscopy
- Nuclear magnetic resonance spectroscopy
- Mass spectrometry
Applications of Constitutional Isomerism: Which Of The Following Molecules Are Constitutional Isomers
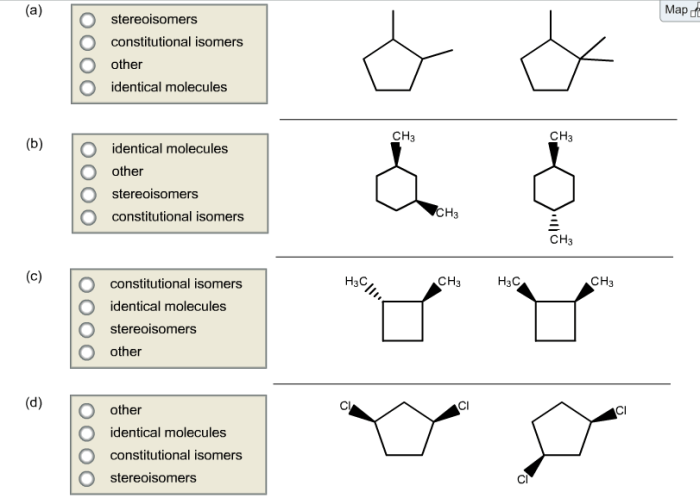
Constitutional isomerism has a number of applications in various fields. These applications include:
- Drug design
- Materials science
- Chemical synthesis
FAQ Insights
What is the key difference between constitutional isomers and stereoisomers?
Constitutional isomers differ in the connectivity of their atoms, while stereoisomers have the same connectivity but differ in the spatial arrangement of their atoms.
How can we determine if two molecules are constitutional isomers?
By comparing their molecular formulas and structural formulas, we can identify whether two molecules are constitutional isomers.
What are some practical applications of constitutional isomerism?
Constitutional isomerism plays a crucial role in drug design, materials science, and chemical synthesis, allowing scientists to tailor the properties of molecules for specific applications.
