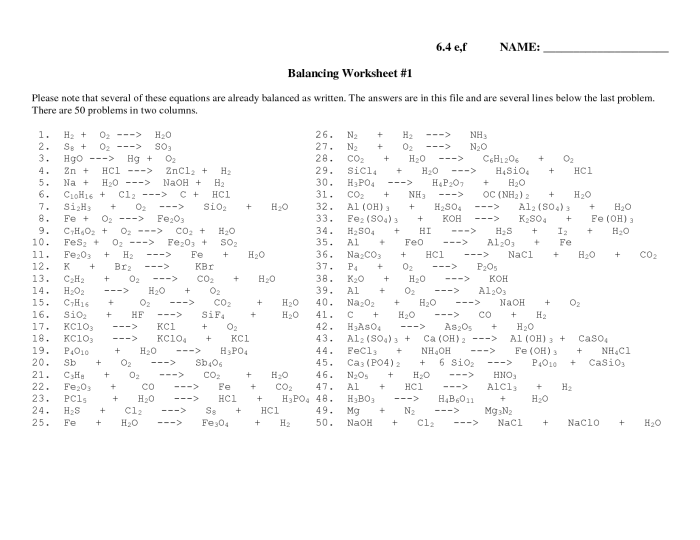
The Intro to Balancing Equations Worksheet provides a comprehensive overview of the fundamental principles and methods involved in balancing chemical equations. This essential skill is crucial for understanding and predicting the outcomes of chemical reactions, making it indispensable for students and practitioners in chemistry.
This worksheet introduces the concept of balancing equations, emphasizing its importance in stoichiometry calculations and chemical engineering. It presents a step-by-step guide to various balancing methods, including the oxidation-reduction method, half-reaction method, and matrix method.
Introduction to Balancing Equations: Intro To Balancing Equations Worksheet
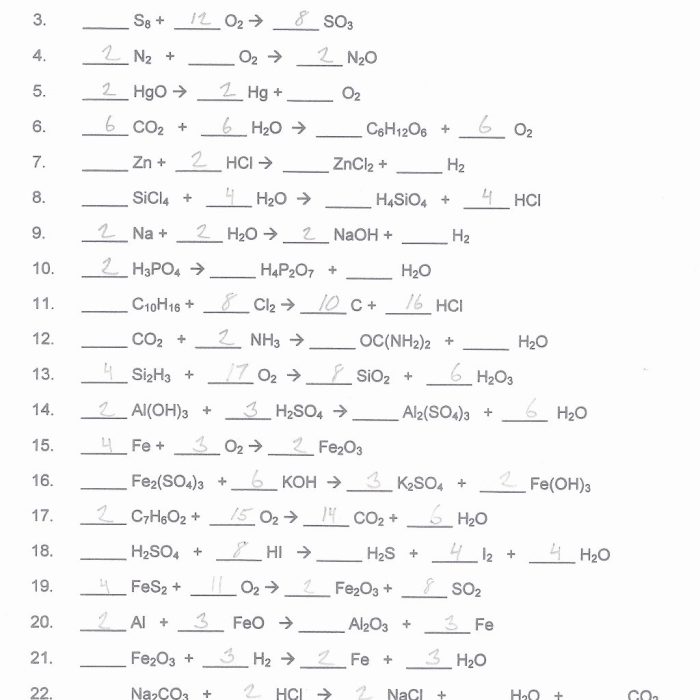
Balancing equations is a crucial step in understanding chemical reactions. It ensures that the number of atoms of each element is the same on both sides of the equation, thereby adhering to the law of conservation of mass. Balancing equations is essential for various chemical calculations and predicting reaction outcomes.
The basic principles of balancing equations involve identifying the reactants and products, counting the number of atoms of each element, and adjusting the coefficients in front of the chemical formulas to make the number of atoms equal on both sides.
Methods for Balancing Equations, Intro to balancing equations worksheet
There are several methods for balancing equations, including:
- Oxidation-Reduction Method:This method is suitable for redox reactions, where electrons are transferred between reactants. It involves identifying the oxidation and reduction half-reactions and balancing them separately before combining them to form the overall balanced equation.
- Half-Reaction Method:This method is similar to the oxidation-reduction method but is more general and can be used for any type of reaction. It involves splitting the reaction into two half-reactions, balancing each half-reaction, and then combining them to form the overall balanced equation.
- Matrix Method:This method is used for complex equations with multiple reactants and products. It involves setting up a matrix of coefficients and solving it using algebraic techniques to find the balanced coefficients.
Examples and Practice Problems
Example 1:Balance the following equation:
Al + 3HCl → 2AlCl3+ 3H 2
Balanced Equation:
Al + 6HCl → 2AlCl3+ 3H 2
Example 2:Balance the following equation:
Fe2O 3+ CO → Fe + CO 2
Balanced Equation:
Fe2O 3+ 3CO → 2Fe + 3CO 2
Practice Problems:
| Unbalanced Equation | Balanced Equation |
|---|---|
| C6H12O6 → C2H5OH + CO2 | C6H12O6 → 2C2H5OH + 2CO2 |
| NH3 + O2 → NO + H2O | 4NH3 + 5O2 → 4NO + 6H2O |
Applications of Balancing Equations
Balanced equations are essential in various applications, including:
- Stoichiometry Calculations:Balanced equations allow us to determine the quantitative relationship between reactants and products, enabling us to calculate the amounts of reactants and products involved in a reaction.
- Predicting Reaction Outcomes:By balancing equations, we can predict the products and their relative amounts, providing valuable insights into the reaction’s outcome.
- Chemical Engineering:Balanced equations are used in chemical engineering to design and optimize chemical processes, ensuring efficient and safe operation.
Popular Questions
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
What are the different methods for balancing chemical equations?
There are several methods for balancing chemical equations, including the oxidation-reduction method, half-reaction method, and matrix method. Each method has its own advantages and is suitable for different types of reactions.
Why is it important to balance chemical equations in stoichiometry calculations?
Balancing chemical equations is crucial in stoichiometry calculations because it allows us to determine the exact amounts of reactants and products involved in a reaction. This information is essential for predicting reaction yields and designing efficient chemical processes.